It started with a mystery.
Why do some of the world’s most advanced cancer drugs… simply stop working?
Doctors had seen it happen countless times. Patients with hope in their eyes start treatment, tumors shrink, the charts look better… and then, without warning, the cancer fights back.
The drugs didn’t fail because they were bad. They failed because cancer had a secret trick. A biological cloaking device that told the immune system: “Don’t attack me.”
For years, researchers tried to crack that code. Billions of dollars went into a class of drugs called immune checkpoint inhibitors. Medicines that “unlock” the immune system so it can recognize and kill cancer cells.
Some worked wonders. But the success rate was still frustratingly low, especially for aggressive cancers.
Then came a new idea.
What if you could take today’s best immune therapies… and supercharge them by blocking that “Don’t Eat Me” signal?
What if you could make the immune system’s attack dogs — the macrophages — recognize and destroy tumors the moment they see them?
One small U.S. biotech has spent years perfecting this approach. It claims to have solved the biggest safety problem that sank earlier attempts. The side effects that came when these drugs attacked healthy blood cells.
And now, with a safer, more targeted version in hand… they’re preparing to take it into human trials.
The company’s name? Liminatus Pharma (NASDAQ: LIMN).
Why This Approach Is Attracting Billion-Dollar Deals
Big pharma isn’t blind to the potential of this “Don’t Eat Me” signal breakthrough.
When a company shows even early success in safely targeting it, the deals can be massive:
- AbbVie (NYSE: ABBV) — In 2020, AbbVie signed an exclusive licensing deal with I‑Mab for a CD47 antibody. The structure: $200 million upfront plus potential milestones totaling up to $1.74 billion.1
- Trillium Therapeutics — Acquired by Pfizer for $2.26 billion after showing promising early results in this same pathway.2
- I-Mab Biopharma (NASDAQ: IMAB) — Still in trials, yet commands a $1.02 billion market valuation thanks to its own CD47 program.3
These aren’t one-off flukes. They show a pattern:
When a new therapy can improve the performance of existing cancer treatments — especially blockbuster immune checkpoint inhibitors — the biggest names in the industry will pay, and they’ll pay big.
That’s what makes Liminatus Pharma (NASDAQ: LIMN) so intriguing right now.
They believe their next-generation CD47 therapy can do what others couldn’t — deliver the cancer-killing benefits without the dangerous side effects that derailed earlier competitors.
If they’re right, this isn’t just another biotech story. It’s a chance to be positioned before a technology shift that could lead to licensing deals, acquisitions, or partnerships worth billions.
SUBSCRIBE FOR TRADING INSIGHTS AND ALERTS. STAY AHEAD.
Get investment opportunities before the rest of the market in real-time.

Get this company's corporate presentation now. Subscribe to download!
Over 120,000 subscribers
Why This Could Potentially Be One of the Fastest-Running Biotech Stocks on Nasdaq
In biotech, news can change everything overnight.
On June 9, Liminatus Pharma (NASDAQ:LIMN) traded at $26.68.
Today, it’s a fraction of that.
With just 12.46 million shares in the public float4, it doesn’t take much volume for the price to move — and move fast.
Add in the fact that the company just revealed an unusual, headline-grabbing plan, a strategic review to create a separate $500 million digital asset investment vehicle.5 It’s got the perfect recipe for market-moving headlines.
This new arm would operate independently of the cancer drug pipeline, aiming to raise capital through private placements, convertible instruments, and equity lines, potentially giving the company access to hundreds of millions in non-dilutive funding, while keeping its core biotech work fully funded.
If executed, it would be one of the boldest treasury strategies ever attempted by a Nasdaq-listed biotech and could attract entirely new classes of investors.
When you combine the pipeline potential, M&A precedent, and capital innovation, you get a setup that’s rare on Wall Street.
Here’s why some investors are circling this story right now:
8 Reasons
This Tiny Nasdaq Biotech Could Be One Breakthrough – or One Headline – Away From a Big Move
1
Next-Gen CD47 Cancer Drug — Designed to supercharge today’s best immune therapies while avoiding the blood cell safety issues that sank earlier attempts.
2
Billion-Dollar Precedent — Similar assets have sold or been licensed for $1–$4.9 billion to major pharma players like Pfizer and AbbVie.
3
Low Float — Only 12.46M shares outstanding Liminatus Pharma’s (NASDAQ:LIMN) float means the stock can run quickly on news.6
4
Fast-Run Potential Proven — The stock was $26.68 as recently as June 9, proving it can move sharply when momentum hits.
5
Pipeline Beyond One Drug — Includes additional immune-oncology programs and an artificial blood joint venture for emergency and military use.
6
Bold Capital Strategy — July 25 announcement of a potential $500M digital asset investment vehicle to fund operations without heavy dilution.
7
Near-Term Catalysts — Human trials for the CD47 therapy are on the horizon, with partnership potential built into the model.
8
M&A and Partnership Appeal — If the technology delivers in trials, Liminatus Pharma (NASDAQ:LIMN) could become a prime acquisition target for big pharma looking to enhance their immunotherapy portfolios.
Press Releases
- Liminatus Pharma Inc. Evaluates Formation of Digital Asset Investment Vehicle Targeting Up To $500 Million in Capital Strategies
- Liminatus Pharma Inc. Takes Next Step in Evaluating Digital Asset-Linked Capital Strategy
- Liminatus Pharma, Inc. Initiates Strategic Review of Blockchain-Integrated Treasury Strategy
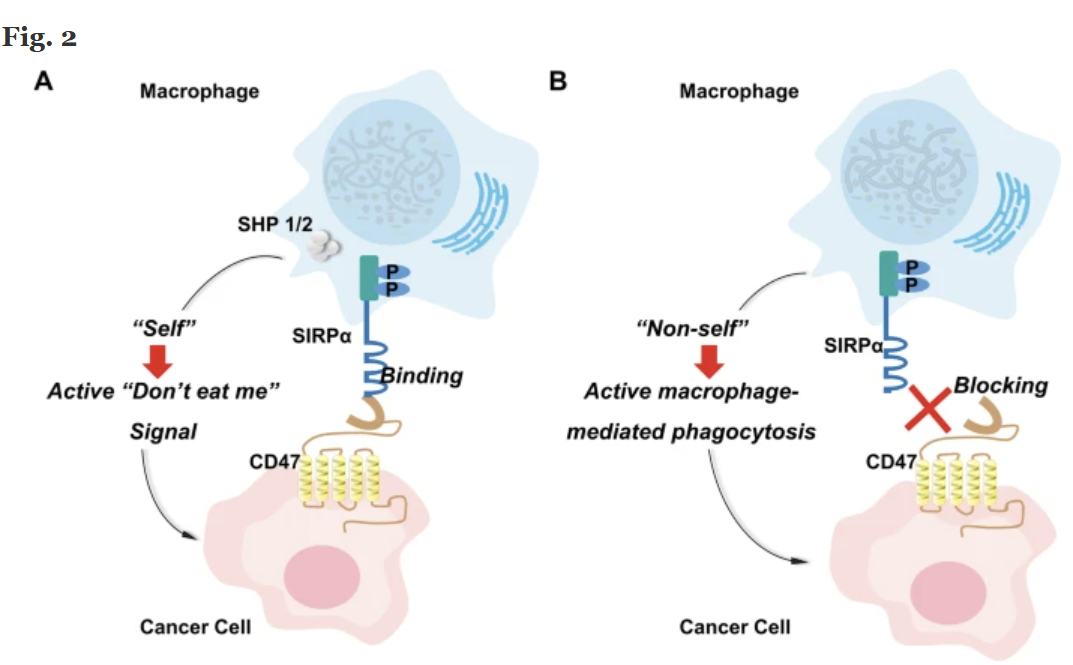
How This “Don’t Eat Me” Switch Works And Why This Company Thinks It Can Do It Safer
Think of your immune system like a neighborhood watch.
Its job is to look for anything suspicious which in this case are cancer cells — and call in the “cleanup crew” to take it out.
One of the immune system’s most powerful cleanup crews are called macrophages. They’re like the garbage trucks of your body, finding and “eating” dangerous cells.
But cancer has learned a trick.
It puts up a “Don’t Eat Me” sign on its surface. Scientists call that sign CD47. When macrophages see the CD47 signal, they pass right by leaving the cancer alone to grow and spread.
Now here’s where it gets exciting:
If you can block that CD47 signal, you take away the cancer’s disguise. Suddenly, the macrophages know exactly what to do by surrounding the tumor and destroying it.
The problem?
Earlier drugs that tried this caused serious side effects. That’s because CD47 isn’t just on cancer cells, it’s also on healthy red blood cells and platelets.
Blocking it everywhere sometimes caused dangerous drops in blood counts, and several big programs were shut down.
Liminatus Pharma’s (NASDAQ:LIMN) scientists think they’ve solved it.
Their CD47 drug is designed to target tumor and immune cells only, while barely touching red blood cells or platelets. In preclinical safety studies, they saw no major blood-related side effects, a key difference that could make all the difference in winning FDA approval.
If they’re right, this isn’t just about beating one type of cancer, it’s about making today’s leading cancer drugs work better, faster, and for more people.
SUBSCRIBE FOR TRADING INSIGHTS AND ALERTS. STAY AHEAD.
Get investment opportunities before the rest of the market in real-time.

Get this company's corporate presentation now. Subscribe to download!
Over 120,000 subscribers
The Size of the Prize: Two Multi-Billion-Dollar Markets
In biotech investing, size matters.
The bigger the market, the greater the upside if a company can capture even a small slice of it.
That’s why what Liminatus Pharma (NASDAQ:LIMN) is targeting is so remarkable — it’s not one giant market… it’s two.
The first is one of the hottest areas in all of medicine: immuno-oncology, where the immune system is trained to hunt and destroy cancer.
The second is a sector that’s been on military wish lists and hospital procurement plans for decades: artificial blood, a technology that could save lives anywhere from a battlefield to an ambulance.
Both markets are projected to explode in value over the coming years. And with its next-generation CD47 cancer therapy and its artificial blood joint venture, Liminatus Pharma (NASDAQ:LIMN) has a shot at competing in both.
1. Immuno-Oncology / CD47 Therapies
Cancer drugs that enlist the immune system have changed the treatment landscape — and the revenues of the companies behind them. The two best-known PD-1 inhibitors bring in tens of billions annually.8,9
But scientists now believe that combining PD-1 drugs with CD47 blockers could make treatments even more effective. That’s where Liminatus Pharma (NASDAQ:LIMN) comes in with its lead candidate, IBA101 — a potentially safer, second-generation CD47 therapy designed to avoid the safety pitfalls that derailed earlier competitors.
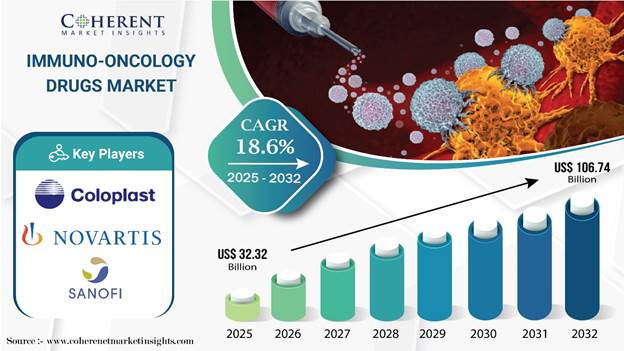
- The immuno-oncology space is booming. Market analysts expect immuno-oncology drugs to grow from $32.3 billion in 2025 to $106.7 billion by 2032, at an 18.6% annual clip. A trend that strongly supports the blockbuster potential of CD47-targeted treatments.10
- The CD47 targeting therapeutics market is expected to grow at a 22.89% compound annual growth rate (CAGR) to reach approximately $18.96 billion by 2034.11
- Big pharma has already moved in: Pfizer paid $2.26 billion to acquire Trillium Therapeutics in 2021 for its CD47 program, and AbbVie signed a $2 billion licensing deal with I-Mab in 2020 for its own CD47 antibody.
This is the same playing field Liminatus Pharma (NASDAQ:LIMN) is stepping onto. A space where billion-dollar buyouts have already proven the commercial potential.
2. Artificial Blood / Oxygen Carriers
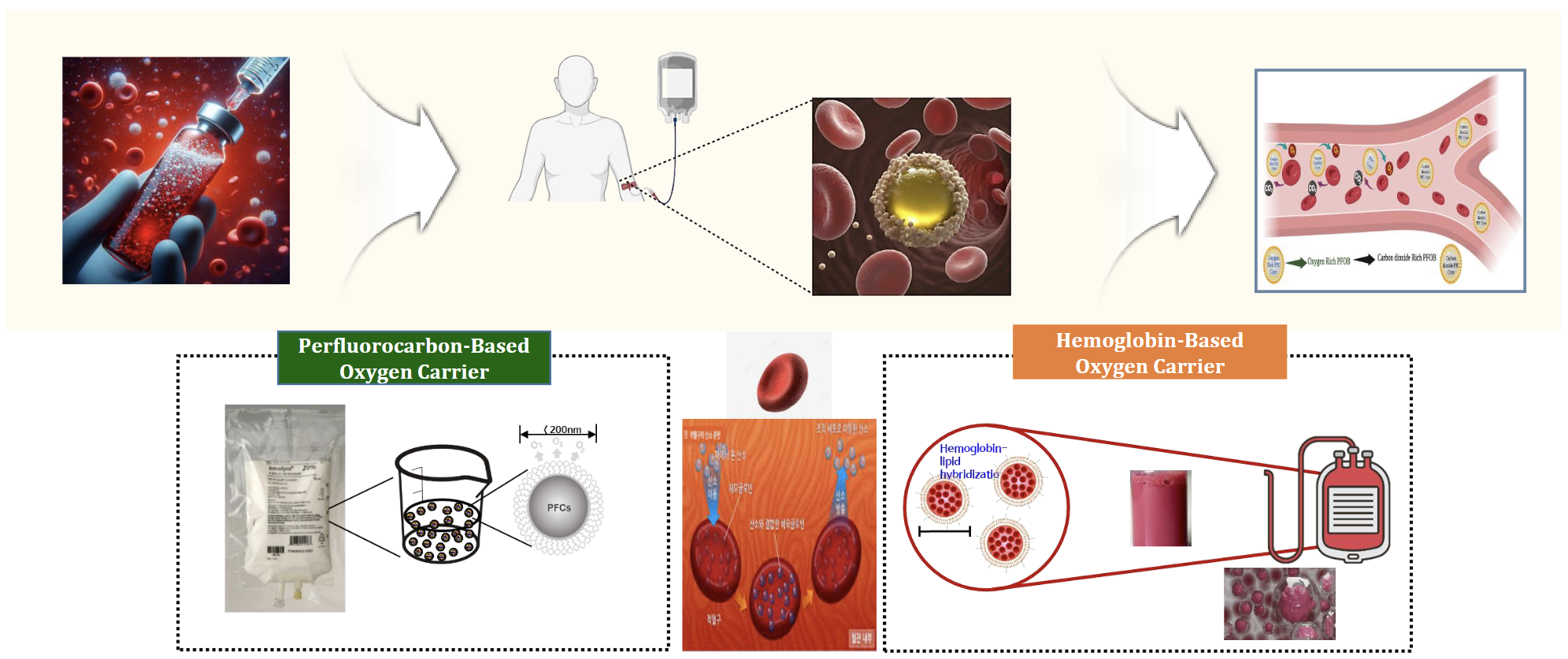
Now imagine a world where hospitals never run short of blood.
Where medics can stabilize trauma patients in minutes without worrying about blood type matching, refrigeration, or supply shortages.
That’s the promise of artificial blood. For decades, researchers have been chasing a viable, safe alternative to donor blood. The potential buyers aren’t just hospitals, they’re militaries, emergency services, space agencies, and even disaster relief organizations.
Liminatus Pharma (NASDAQ:LIMN), through its artificial blood joint venture, is positioning itself in the center of this future. With backing from the South Korean Ministry of National Defense and advanced R&D partners, the company is moving toward solutions that could change how the world thinks about transfusions.
- The artificial blood market is projected to grow from $3.89 billion in 2019 to $15.4 billion by 2027, a compound annual growth rate of 18.5%.12
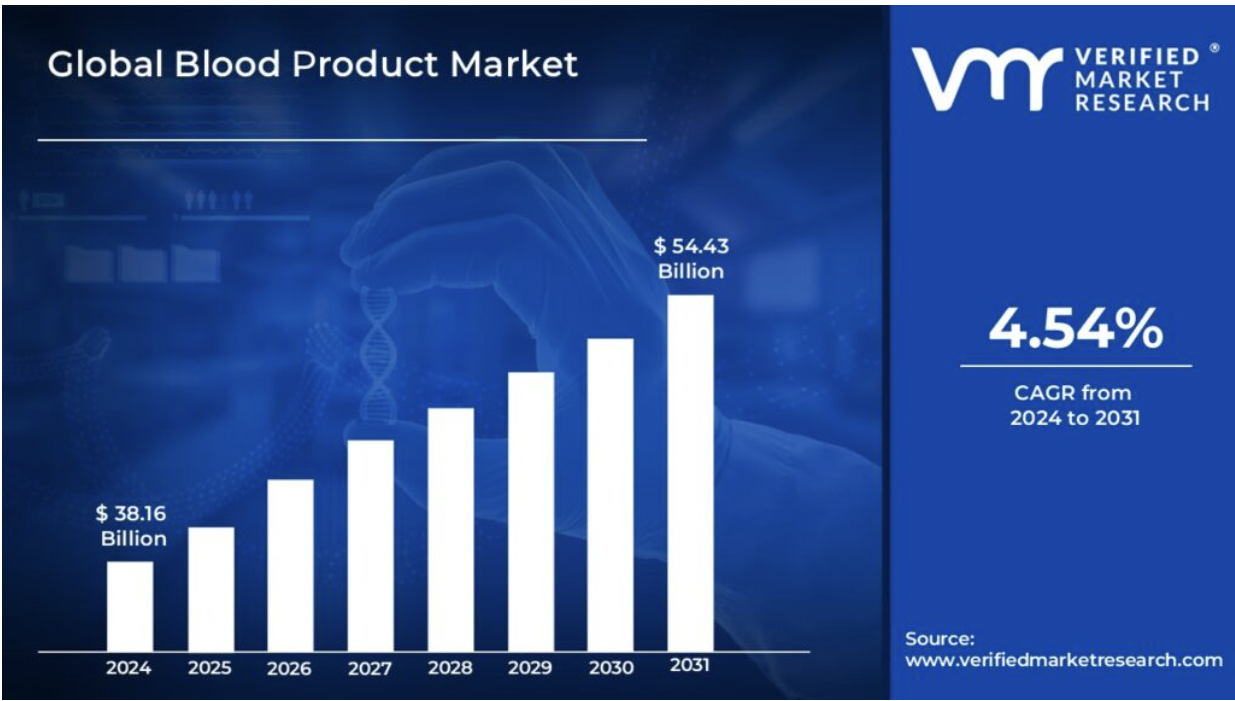
- The global human blood products market, the broader category that includes blood transfusions and related therapies is valued at USD 38 billion in 2025, and is expected to grow to approximately USD 54 billion by 2031.13
Why This Matters for Investors
Biotechs that can move the needle in just one of these markets can transform overnight.
Liminatus Pharma (NASDAQ:LIMN) is aiming at both, giving it two shots on goal in industries where even a fraction of the market translates to billions in potential revenue.
Upcoming Catalysts That Could Light the Fuse
Some biotech stocks drift for years.
Liminatus Pharma (NASDAQ:LIMN) is NOT one of them.
Liminatus is sitting on multiple “binary events”. Moments where a single press release can change the story overnight.
And with just 12.46 million shares in the public float, it doesn’t take much buying pressure to send the price moving fast. We’ve already seen what’s possible.
On June 9, this stock traded at $26.68. In the right conditions, it can run hard and fast.
Now, with regulatory filings, trial launches, and potential partnerships all lined up, the next wave of headlines could put this ticker back on the market’s radar in a big way.
Here’s what’s coming…
Key Catalysts on the Horizon
1. IND Submission for IBA101 (Late 2026)
Liminatus Pharma (NASDAQ:LIMN) is preparing for a major leap into human studies. The company has completed all necessary safety and efficacy testing in non-human primates, conducted under GLP standards at Charles River Laboratories to support the regulatory filings. As a result, they plan to submit Investigational New Drug (IND) applications to both the U.S. FDA and Korea’s Ministry of Food and Drug Safety (MFDS) in the second half of 2026.14 This regulatory green light would open the door to initiating human trials and setting the stage for potentially transformative results.
2. Phase I First-in-Human Trials (Early 2027)
Liminatus Pharma (NASDAQ:LIMN) is rapidly moving from the lab to the clinic. According to its June 2025 disclosures, the company completed all necessary preclinical testing and is now preparing for human trials.
The company has finished GLP toxicology and pharmacology studies in primates, paving the way for regulatory submissions and clinical planning. Site activations and patient screening are expected to begin in early 2027.
Liminatus is collaborating with Professor Se-Hoon Lee at Samsung Medical Center in Seoul. Planning a 3+3 dose-escalation Phase I protocol, with expansion cohorts and combination arms alongside PD-1/PD-L1 therapies to assess safety, dosing, and potential synergy.15
3. Potential Big Pharma Collaborations
The trial design allows for testing alongside existing PD-1 therapies, opening the door to co-development or licensing deals if synergy is seen.
4. $500 Million Capital Strategy Decision
The Board is reviewing the creation of a separate digital asset investment arm. If approved, it could give Liminatus Pharma (NASDAQ:LIMN) a massive funding pool without heavy share dilution — rare in biotech.
5. Exploratory Programs in Inflammation
Early research suggests the CD47 platform could help clear harmful senescent cells in chronic inflammation, adding entirely new markets to the pipeline.
6. Market Reaction to Any Positive Data
Low float, high sensitivity to news, and a history of fast moves mean that even a small positive update could send shares of Liminatus Pharma (NASDAQ:LIMN) sharply higher.
7. FDA Regulatory Visibility
Liminatus is already on MarketBeat’s FDA Events Dashboard, keeping it on the radar of traders and funds who track upcoming drug milestones.16
Meet the Team Steering the Breakthroughs
Behind every biotech breakthrough, there’s a team made of brains, experience—and serious hustle. Liminatus Pharma (NASDAQ:LIMN) has assembled just that:
Adding Depth: The Board of Directors
Liminatus recently expanded its board to include experts capable of scaling science into solutions:
- Philip Lemons, a clinical R&D leader with two decades of experience working globally with institutions like Duke Clinical Research Institute. He’ll help navigate trial design and strategic alliances.20
- Richard Baek, a finance and compliance specialist who ensures the company stays audit-ready and accountable, a must for any emerging public company.21
Why This Team Inspires Confidence
- Scientific Strength + Legal Savvy: With Dr. Yoo and Chris Kim leading the scientific and IP fronts, the pipeline isn’t just smart—it’s protected.
- Financial Competence: Scott Dam and Richard Baek cover the numbers with their fundraising savvy and compliance so investors don’t have to worry.
- Operational & Clinical Credibility: Adding Lemons bridges the lab and the clinic. Exactly what Liminatus needs as they move into human trials.
In plain terms: this is a lean team with heavyweight experience that is built to move fast, navigate bureaucracy, and deliver results.
Low Float. Big Markets. Binary Events Ahead.
The Clock Is Ticking.
In biotech, timing is everything.
And right now, Liminatus Pharma (NASDAQ:LIMN) is sitting on a perfect storm of opportunity. A low 11.87 million share float, two billion-dollar market targets, and multiple binary events that could hit the tape over the next 12–18 months.
We’ve already seen what this stock can do when it catches momentum. On June 9, shares hit $26.68. With the right news, it probably won’t take much for a potential move like that to happen again.
Between its potentially safer CD47 therapy, its military-backed artificial blood program, and a board exploring a $500 million capital strategy, Liminatus Pharma (NASDAQ:LIMN) is positioned for news flow that could wake up the market in a big way.
Biotech investors have learned the hard way that waiting for the press release is often too late. The market tends to price in good news fast, sometimes in a single trading session.
That’s why if you’re watching Liminatus Pharma (NASDAQ:LIMN), you’ll want to be ready before the headlines hit.
The next phase of this story could move quickly and the setup is already in place.
Subscribe here to be the first to get their latest news and updates.
SUBSCRIBE FOR TRADING INSIGHTS AND ALERTS. STAY AHEAD.
Get investment opportunities before the rest of the market in real-time.

Get this company's corporate presentation now. Subscribe to download!
Over 120,000 subscribers












